About Coyne lab
The nuclear pore complex and surrounding nuclear envelope environment function to maintain essential cellular processes including nucleocytoplasmic transport and gene expression. Thus, maintenance of these structures and their individual protein components is essential for continued neuronal function and survival.
We have recently defined a significant injury to the nuclear pore complex and nuclear envelope that contributes to pathophysiological events such as the mislocalization and dysfunction of the RNA binding protein TDP-43 in Amyotrophic Lateral Sclerosis and related neurodegenerative diseases. This early event in disease pathogenesis is initiated by the overactivation/inappropriately sustained activation of the fundamental ESCRT-III nuclear surveillance pathway.
The overall goal of the Coyne lab is to understand the molecular mechanisms that underlie key events in this nuclear pore complex/nuclear envelope injury cascade to disrupt overall nuclear pore complex homeostasis and compromise cellular function to give rise to and/or propagate neurodegenerative disease pathogenesis.
To address these questions, we utilize induced pluripotent stem cell derived neurons (iPSNs), immortalized cell lines, postmortem human tissues, and Drosophila models combined with multiple imaging modalities (super resolution, confocal, live cell, EM), gene editing, and molecular and biochemical technologies.
We are currently recruiting at all levels- undergraduate, graduate, postdoc, and technician. If interested, please contact the PI Alyssa Coyne at acoyne3@jhmi.edu.
Selected Recent Publications:
# co-corresponding author; Coyne Lab Member
Rothstein JD#, Baskerville V, Rapuri S, Mehlhop E, Jafar-nejad P, Rigo F, Bennett F, Mizielinska S, Isaacs A, Coyne AN# (2023) G4C2 targeting antisense oligonucleotides potently mitigate TDP-43 dysfunction in C9orf72 ALS/FTD human neurons, bioRxiv
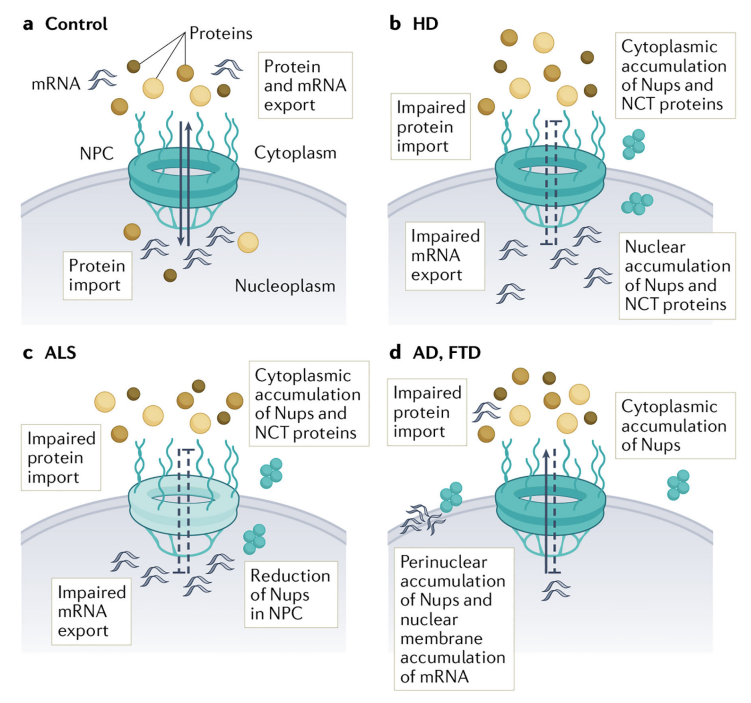
Chandia Cristi A, Rapuri S, Coyne AN (2023) Nuclear pore complex and nucleocytoplasmic transport disruption in neurodegeneration, FEBS Letters, PMID: 37657945
Baskerville V, Rapuri S, Mehlhop E, Coyne AN (2023) SUN1 facilitates CHMP7 nuclear influx and injury cascades in sporadic Amyotrophic Lateral Sclerosis, Brain, PMID: 37639327
Coyne AN# and Rothstein JD# (2021) Nuclear pore complexes: a doorway to neuronal injury in neurodegeneration, Nature Reviews Neurology, PMID: 35488039
Coyne AN and Rothstein JD (2021) The ESCRT-III protein VPS4, but not CHMP4B or CHMP2B, is pathologically increased in familial and sporadic ALS neuronal nuclei, Acta Neuropathologica Communications, PMID: 34281622
Coyne AN#, Baskerville V, Zaepfel BL, Dickson DW, Rigo F, Bennett F, Lusk P, Rothstein JD# (2021) Nuclear accumulation of CHMP7 initiates nuclear pore complex injury and subsequent TDP-43 dysfunction in ALS, Science Translational Medicine, PMID: 34321318
Coyne AN# and Rothstein JD# (2021) Nuclei isolation and super resolution structured illumination microscopy for examining nucleoporin alterations in human neurodegeneration, Jove, PMID: 34570098
Coyne AN and Rothstein JD (2021) Nuclear lamina invaginations are not a pathological feature of C9orf72 ALS/FTD, Acta Neuropathologica Communications, PMID: 33741069
Coyne AN, Zaepfel BL, Hayes L, Fitchman B, Salzberg Y, Luo EC, Bowen K, Trost H, Aigner S, Rigo F, Yeo GW, Harel A, Svendsen CN, Sareen D, Rothstein JD (2020) G4C2 repeat RNA initiates a POM121 mediated reduction in specific nucleoporins in C9orf72 ALS/FTD, Neuron, PMID: 32673563
Full Google Scholar Profile for Alyssa Coyne